Therapeutics and Vaccines R&D
The global burden of antimicrobial resistance requires new antibacterial treatments, especially for the most challenging resistant bacteria.
WHO has developed a list of priority pathogens for which antibiotics are urgently needed and regularly performs an analysis of the clinical and pre-clinical antibacterial pipelines.
Resources are shared here on some of the international partners who are driving investment and innovation in R&D.
|
LEAVING THE LAB Tracking the Decline in AMR R&D Professionals
This document takes a critical look at the unsettling reality of diminishing expertise within the antimicrobial resistance (AMR) research and development (R&D) workforce. It offers a comprehensive review of publicly available data to illuminate the current state of the AMR R&D field.
|
|
Access to Medicines Foundation Antimicrobial Resistance Benchmark Report 2020
The Access to Medicine Foundation has been analysing how pharmaceutical companies tackle access to medicine for more than a decade. This 2020 Antimicrobial Resistance Benchmark has evaluated how the most important players in the antibiotic market are addressing the rise of resistance and the global need for appropriate access to antibiotics. Although there has been progress — it’s hanging by a thread. View the report.
|
|
Global Antibiotic Research & Development Partnership (GARDP)
GARDP was created to ensure that everyone who needs antibiotics receives effective and affordable treatment, no matter where they live and aims to develop five new treatments by 2025 to fight drug-resistant infections, focusing on sexually transmitted infections, sepsis in newborns and infections in hospitalized adults and children. Find out more about their work developing urgently needed treatments for drug-resistant bacteria on the World Health Organization priority pathogens list, as well as for people disproportionately affected by drug resistance, including babies, children and hospitalized people.
|
|
WHO dashboard for antibacterial products in clinical development for priority pathogens
The Global Observatory on Health R&D is a comprehensive source of information and analyses on global health R&D for human diseases. To generate this recently released dashboard (April 2021), WHO analysed the pipeline of antibacterial products (antibiotics and biologicals) that were in phase I-III of clinical development and matched them against the WHO priority pathogens list (PPL), Mycobacterium tuberculosis and Clostridium difficile. Candidate products are reported by type, pathogen category, phase of clinical development, and expected activity against priority pathogens. There is also an assessment of their innovativeness and whether a new chemical entity is involved. The route of administration, antibiotic class and developers are also reported.
|
|
Global AMR R&D Hub Dynamic Dashboard
The Global AMR R&D Hub monitors AMR R&D investments and market interventions. This information is intended for countries, foundations, organisations and initiatives to help set priorities and maximise the impact of resources invested in R&D to mitigate the AMR threat.
The Global AMR R&D Hub's Dynamic Dashboard continuously collects and presents information on AMR R&D investments, products in the pipeline and push and pull incentives across three galleries. It will provide the evidence base to help set priorities and maximize the impact and efficiency of resources and efforts invested into AMR R&D.
|
|
Support for CARB-X-funded AMR therapeutics and vaccines R&D
CARB-X is accelerating global antibacterial innovation by investing in the development of new antibiotics and other life-saving products to combat the most dangerous drug-resistant bacteria. Our projects represent the world’s largest pre-clinical and early development pipeline of antibiotics and other therapeutics, diagnostics, microbiome and vaccines. This document highlights how CARB-X can provide support for CARB-X-funded researchers developing AMR therapeutics and vaccines at all stages of R&D.
|
|
AMR Industry Alliance
The AMR Industry Alliance brings together biotech, diagnostics, generics and research-based pharmaceutical companies, to drive and measure industry progress to curb antimicrobial resistance.
|
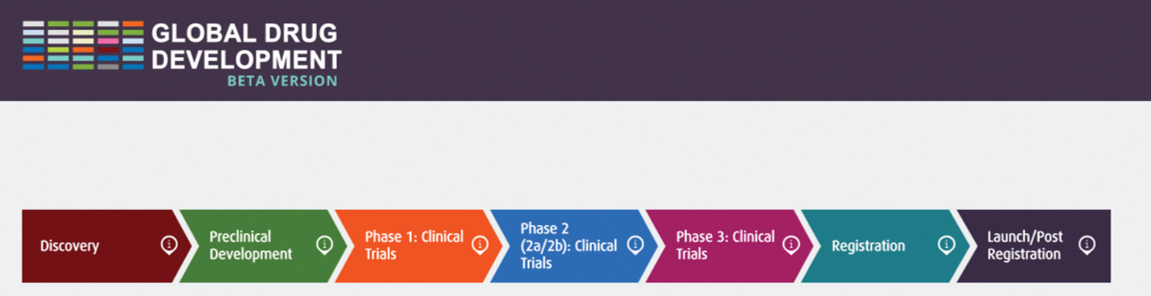
For guidance on drug development, visit the Global Drug Development Process Map - an interactive process map that takes you through the various stages of therapeutic drug development. Access more information on how to use this tool.